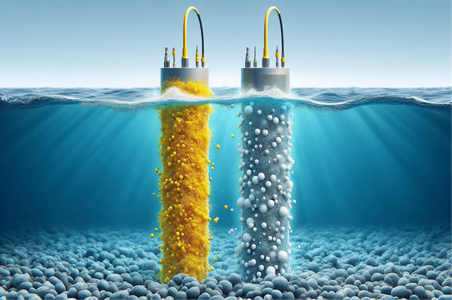
Oceans and seas cover most of the Earth’s surface, and their bottoms are home to many minerals. Their waters also contain uranium ions, albeit in very small amounts. However, if we could extract these ions, they would become a highly sustainable fuel source for generating electricity through nuclear reactors.
Uranium is the most commonly used metal in nuclear reactors. It is extracted from rocks, and the Earth is estimated to contain 8 million tons of uranium. The Nuclear Energy Agency estimates that 4.5 billion tons of uranium are dissolved in the seas and oceans in the form of uranium ions. This makes the ocean’s uranium reserves about 500 times greater than those found on land.
Scientists and engineers have been investigating methods to extract uranium from seawater for decades. This requires developing selective materials that can absorb uranium and retain it. An electrochemical process is also used to attract uranium ions to these absorbent materials.
These methods face challenges in developing good absorbent materials that can retain uranium and work for a reasonable period. They also face difficulty in providing sustainable and inexpensive energy for the electrochemical process.
However, researchers continue to work on developing these materials. Wind or solar energy could be used as a sustainable source to power the electrochemical process. But these methods do not seem economically viable, as they are still very costly and difficult to implement, which keeps them in the research phase for now.
Research and Advancements
Some of the most prominent research in developing these technologies includes:
- Last year, Chinese researchers developed a material that can be used in electrochemical extraction and attracts uranium ions more efficiently than previous methods. This material causes uranium to accumulate on a coated fabric connected to an electrode. Australian researchers have also developed another material that absorbs only uranium and does not absorb other salts and minerals.
- Recently this year, a Chinese team also developed a less expensive absorbent material than its predecessors, but it does not absorb only uranium; it absorbs ions of another substance along with it, making the process of separating uranium after extraction difficult and complex.
It seems that designing a suitable material for uranium absorption is the key. This material must be inexpensive on the one hand and able to work for long periods on the other. Achieving these goals is still a long way off.
Conclusion
Despite the low concentration of uranium in seawater, the total quantity is vast. There are many challenges that must be overcome before its extraction becomes possible and economically viable. Research continues to develop effective methods for its extraction. If successful, the oceans and seas will become a new and vast source of nuclear fuel, providing us with cheap electricity for hundreds of years to come.