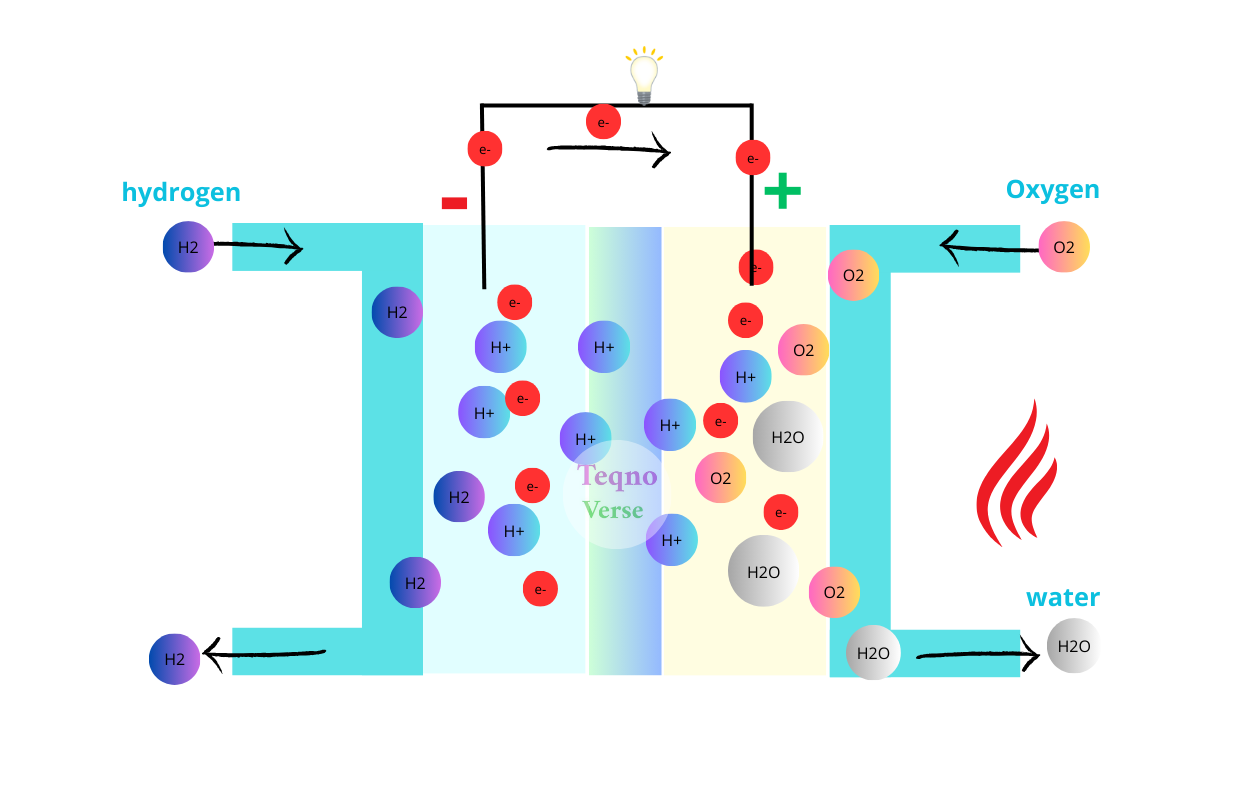
Liquid hydrogen can produce three times the energy of the same weight of gasoline or diesel, but the challenge lies in its difficult transportation and storage. Storing hydrogen requires pressure-resistant containers, and it is also unsafe and flammable.
Researchers at RIKEN Center for Emergent Matter Science (CEMS) in Japan have discovered a compound that can be used to store ammonia, making it safer and easier to transport.
Each ammonia (NH3) molecule contains three hydrogen atoms and can therefore be used to store hydrogen and release it when needed.
Storing ammonia is also complex and is done by liquefying it at low temperatures (-33 degrees Celsius) in pressure-resistant containers.
The new discovery involves the use of perovskite, a material with a distinctive repeating crystalline structure, which can easily store ammonia and allow for its easy and complete recovery by simply heating it to 50 degrees Celsius.
Perovskite ethylammonium lead iodide (EAPbI3) reacts with ammonia at room temperature and pressure, transforming into a two-dimensional layered structure called lead iodide hydroxide, or Pb(OH)I.
Ammonia is thus stored within the layered structure through a chemical transformation, which is a much cheaper process than liquefaction at -33 degrees Celsius in pressurized containers. More importantly, the process of recovering the stored ammonia is also simple.
The researchers have thus found a cheap and safe way to store hydrogen-carrying ammonia, boosting technologies aimed at transitioning to clean energy.
This discovery was published in the Journal of the American Chemical Society on July 10th.