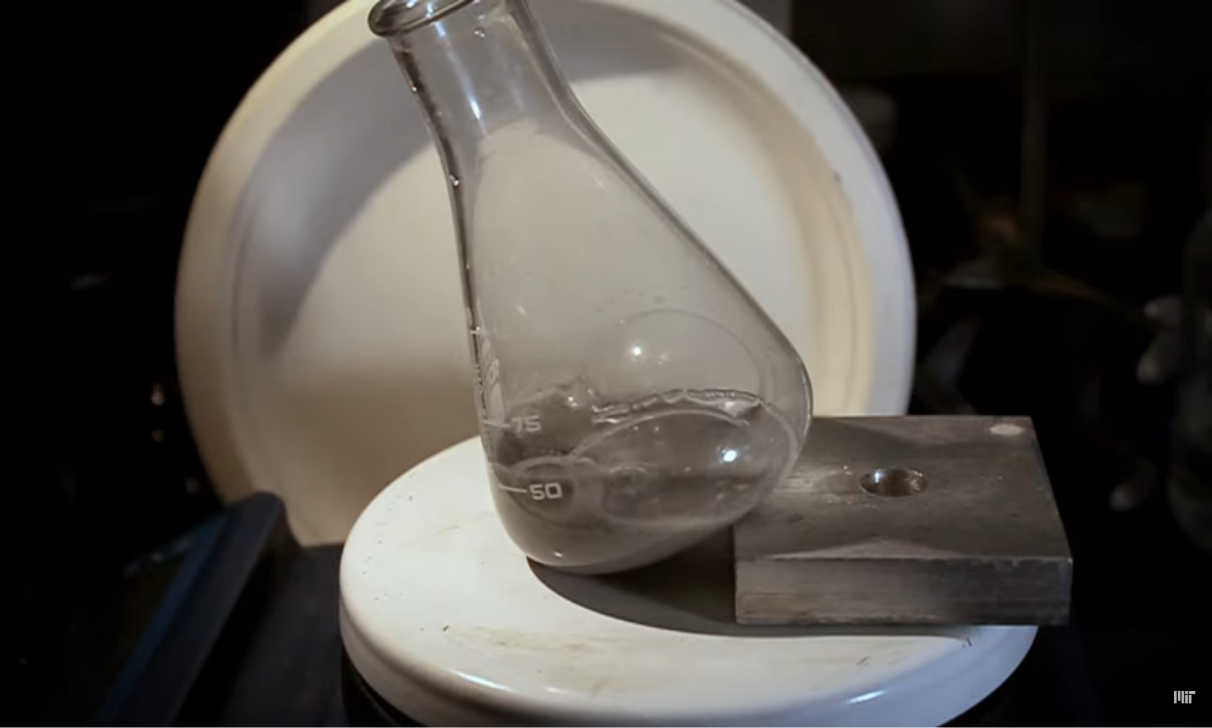At the Center for Nanoparticle Research at the Institute for Basic Science (IBS) at Seoul National University in South Korea, a team of researchers has developed an innovative system that uses sunlight to convert polyethylene terephthalate (PET) waste, the common plastic material in water and soda bottles, into clean hydrogen.
This innovation addresses two major environmental challenges simultaneously. First, the problem of disposing of plastic waste, which is one of the most complex pollutants due to its slow decomposition and accumulation in oceans and landfills. Second, it contributes to the production of clean hydrogen fuel, considered a promising future energy source because it emits no carbon when used. It can power cars, generate electricity, and support heavy industries, making it an ideal alternative to fossil fuels.
The device consists of a photocatalyst and a hydrogel. The photocatalyst is a special material that reacts with sunlight to stimulate chemical reactions, while the hydrogel, a water-absorbing gel-like material, keeps the photocatalyst afloat on the surface to ensure maximum absorption of sunlight. This floating system is placed in a solution containing pre-treated plastic waste to facilitate its decomposition.
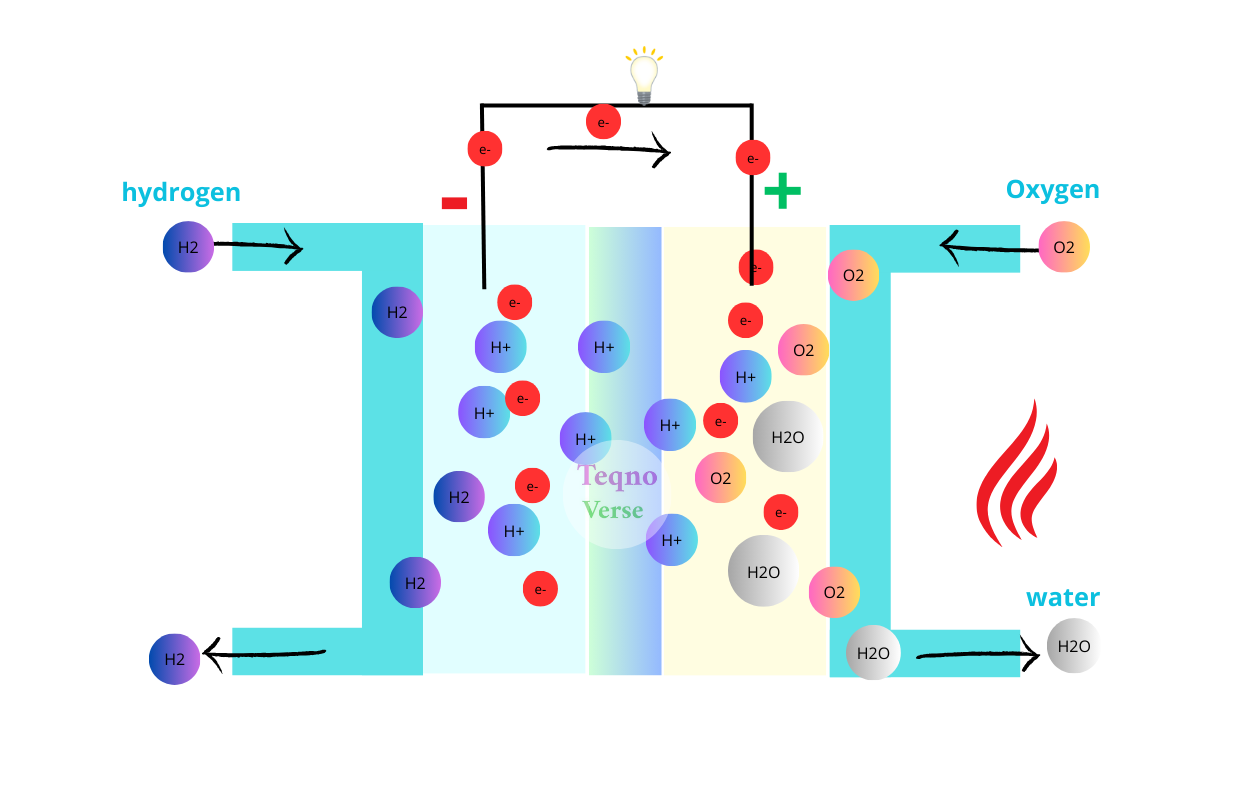
When the device is exposed to sunlight, the photocatalyst absorbs photon energy and releases electrons, triggering a series of chemical reactions. These reactions break down plastic molecules into useful compounds such as ethylene glycol, which is used in antifreeze, and terephthalic acid, which can be reused to make new plastic. Simultaneously, the process produces clean hydrogen gas that can be collected and used as fuel.
This system is distinguished by the fact that it does not require an expensive external power source, as it relies entirely on sunlight. It can also operate effectively even in seawater. The device was developed with a precise engineering design that considers real-world operating conditions outside the laboratory, as it is coated with a protective layer that shields it from environmental factors and temperature changes, allowing it to operate efficiently outdoors.
In a practical test, a one-square-meter device was placed to float on the surface of a solution of PET bottle waste under natural sunlight. The system successfully produced clean hydrogen efficiently without the need for additional electricity or heat, and its performance remained stable for over two months, a remarkable achievement in the field of photocatalysis technologies.
Economic simulations and scalability studies show that this technology can be expanded to areas of up to 10 or 100 square meters, heralding the potential for large-scale, low-cost hydrogen production. Thus, the innovation not only opens new horizons for clean energy but also transforms plastic waste from an environmental burden into a valuable energy resource.
This research was published in the journal Nature Nanotechnology.