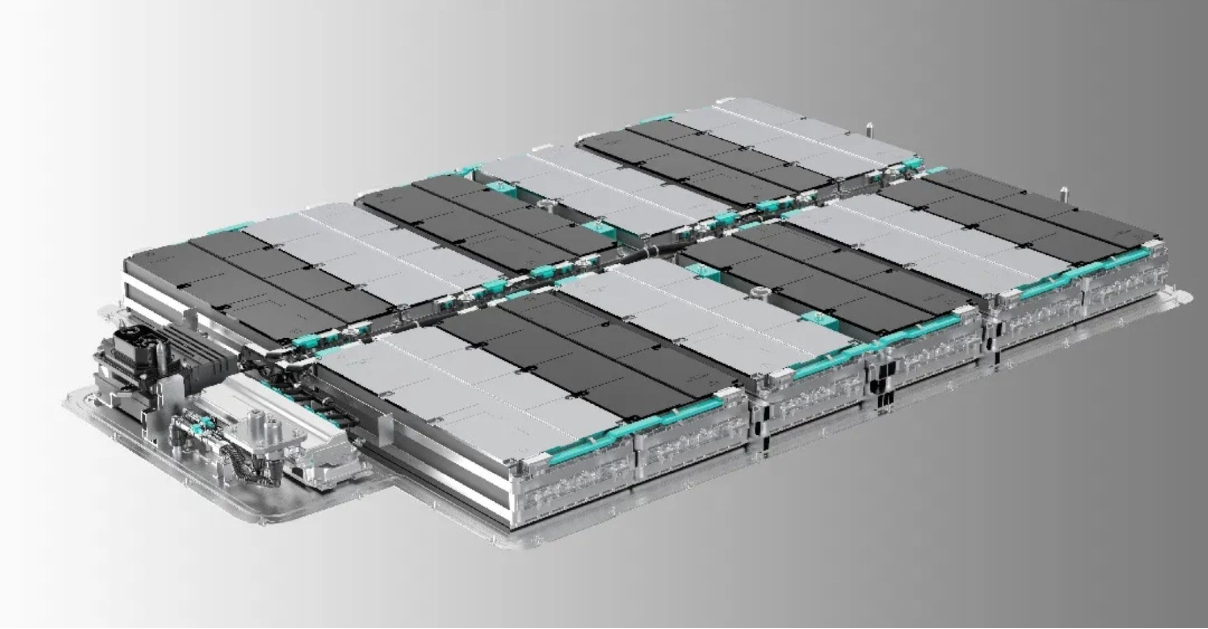
Sodium-ion batteries work on the same principle as lithium-ion batteries, which are found in most electronic devices and electric vehicles. Therefore, replacing them would not require any change in the design or operation of any device. Despite being cheaper than lithium, they are still not widely used.
The low cost of sodium-ion batteries is due to the abundance and ease of extraction of their main component, sodium, compared to lithium, which is expensive, difficult to extract, and environmentally damaging. Sodium is abundant in salt, hence the name “salt batteries”.
In addition to their low cost, sodium-ion batteries are also safer, can withstand higher temperatures, are not prone to fire when overheated, and are environmentally friendly, as the extraction of sodium does not cause environmental damage.
However, sodium (salt) batteries suffer from the problem of low energy density compared to lithium. To store the same amount of energy, larger and heavier sodium-ion batteries are needed. This makes them unsuitable for use in portable devices and cars, where the goal is to reduce weight and increase efficiency. Lithium batteries are still the leader in this field.
Despite this, research is still ongoing to develop materials for sodium-ion batteries that can store more energy by improving the materials of the negative and positive electrodes and the electrolyte between them. Given their low price and energy density, they could be suitable for electric scooters and small cars that do not require high energy density and where weight is not an operational constraint.
Some companies have already started to equip their vehicles with these batteries, such as BYD, which has launched the affordable Seagull car with a sodium-ion battery, and the JAC Group, supported by Volkswagen, which has launched two cars powered by sodium-ion batteries, the Sehol E10x and the Yiwei 3.