Hydrogen fuel cells are devices that convert hydrogen into electrical energy through a process known as an electrochemical reaction. This offers a clean and efficient method for power generation. Today, these cells are used in all hydrogen-powered vehicles available on certain markets, with prominent examples including the Toyota Mirai, Honda Clarity Fuel Cell, and Hyundai Nexo.
How Hydrogen Fuel Cells Work ?
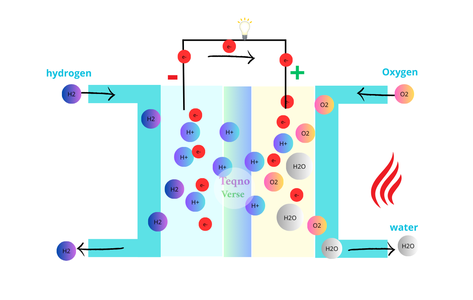
A fuel cell comprises a negative electrode (anode), a positive electrode (cathode), and the electrolyte which is a substance that allows the flow of protons between the anode and cathode while preventing the electrons. Oxygen is typically supplied to the cathode from the surrounding air, while hydrogen gas is fed to the anode from the vehicle’s fuel tank.
At the anode, hydrogen molecules split into hydrogen ions (protons) and free electrons. This separation is facilitated by catalysts, often platinum, which coat the anode and the cathode. The hydrogen ions migrate through the electrolyte membrane to the cathode, while the electrons flow through an external circuit, generating an electric current.
At the cathode, hydrogen ions combine with oxygen and electrons from the external circuit to form water vapor. Any excess hydrogen is recycled.
Consequently, the cell produces electricity as its primary output, along with water vapor and heat as byproducts. Unlike combustion engines, it doesn’t release any harmful pollutants. Moreover, it offers higher efficiency compared to combustion engines, as less energy is wasted during the energy conversion process (from chemical to electrical).
Since each individual cell produces a relatively small amount of energy (typically 0.7 volts), multiple cells are connected in series to form a stack that can generate sufficient power to operate motors or electrical devices.
Types of Hydrogen Fuel Cells
There are various types of hydrogen fuel cells, primarily differentiated by the material of the membrane separating the electrodes:
- Polymer Electrolyte Membrane (PEM) fuel cells: These use a polymer membrane and are widely employed due to their efficiency and ability to operate at ambient temperatures (<100). They are lightweight and compact but necessitate the use of expensive platinum as a catalyst.
- Alkaline Fuel Cells (AFC): Using potassium hydroxide as the membrane, AFCs are suitable for space applications and have a longer lifespan. However, they are more costly to produce and less efficient than PEMs.
- Phosphoric Acid Fuel Cells (PAFC): Employing a molten carbonate salt mixture as the membrane, PAFCs are used for stationary power generation. They are highly reliable but require high operating temperatures (over 600 degrees Celsius).
- Solid Oxide Fuel Cells (SOFC): With a solid ceramic membrane, SOFCs offer high efficiency but demand even higher operating temperatures (800 degrees Celsius).
The Most Commonly Used
Polymer Electrolyte Membrane (PEM) fuel cells are the most prevalent type of hydrogen fuel cell, powering all hydrogen-powered cars, buses, and heavy-duty trucks in operation today.
PEM cells excel at converting the chemical energy in hydrogen into electrical energy. They are lighter and more compact than other types, allowing them to generate more power per unit of weight and volume.
Ongoing research aims to enhance their performance, increase efficiency, and find more affordable and sustainable alternatives to the platinum catalyst.