Thermochemical hydrogen production methods, while highly promising, necessitate high operating temperatures, significantly increasing their cost relative to alternative hydrogen production approaches.
Overcoming this temperature challenge could position thermochemical methods as highly efficient and cost-effective hydrogen production solutions. These methods leverage inexpensive metal oxides, such as iron oxide or cerium oxide. When heated to 1500°C, these oxides release oxygen. Subsequent exposure to water facilitates the regeneration of the oxide, with the water molecule donating its oxygen and releasing hydrogen as a byproduct.
To address this challenge, the POSTECH team developed a novel technique utilizing microwave energy, the same energy source employed in household microwave ovens. The researchers discovered that microwave energy could significantly lower the reduction temperature of Gd-doped ceria (CeO2), a benchmark material for hydrogen production, to below 600°C, reducing the temperature requirement by approximately 60%.
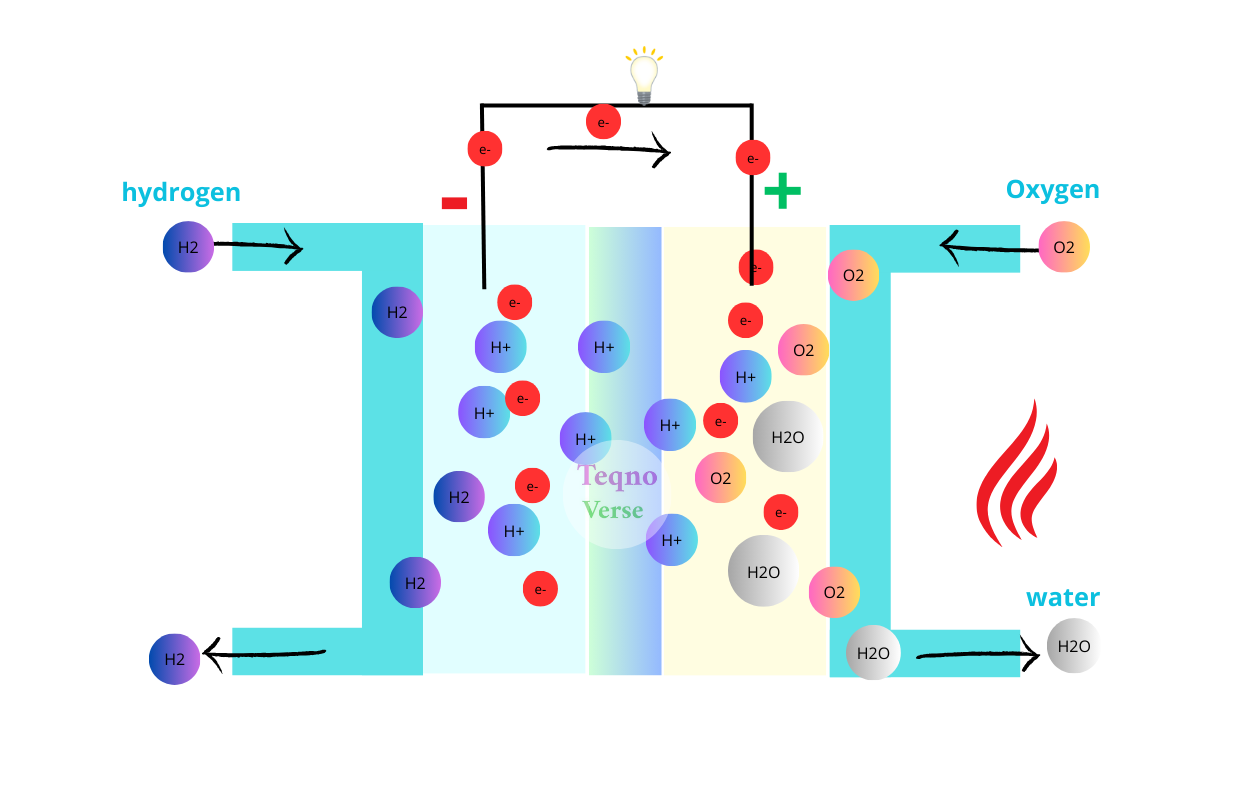
This process significantly accelerates the formation of oxygen vacancies in cerium, which are crucial for water splitting into oxygen and hydrogen. Microwave energy effectively substituted 75% of the required thermal energy, significantly enhancing the process’s efficiency, speed, and cost-effectiveness.
The research was published in the Journal of Materials Chemistry A and is expected to make a significant impact on the commercial production of hydrogen using thermochemical methods.