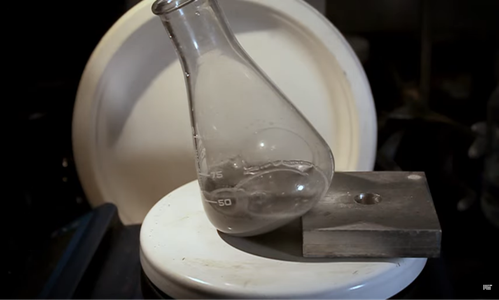Scientists at the Massachusetts Institute of Technology (MIT) have developed a groundbreaking method to produce hydrogen fuel using a surprising combination of materials: seawater, aluminum, and coffee.
Pure aluminum, commonly found in soda cans and other sources, reacts with water after being broken into small, pebble-like pieces. This reaction produces hydrogen gas and aluminum hydroxide. However, a natural oxide layer that forms on the aluminum’s surface when exposed to oxygen in the air or water typically hinders or prevents this reaction from occurring.
To overcome this obstacle, the researchers employed a rare metal alloy composed of gallium and indium. This alloy effectively removes the oxide layer, allowing the aluminum to react with water and generate hydrogen gas. Unfortunately, the high cost of this alloy makes this approach economically unfeasible unless a method for recovering and reusing the alloy can be found.
To address this issue, the team turned to seawater. Seawater can dissolve the alloy, making it possible to recover and reuse it. However, the reaction between aluminum and seawater is significantly slower than with pure water. To accelerate this process, the researchers made a surprising discovery: coffee grounds can dramatically increase the reaction rate. The active ingredient in coffee, imidazole, is the responsible for this effect by penetrating the oxide layer on the aluminum and exposing the underlying metal to water.
This breakthrough opens up new possibilities for the production of hydrogen fuel. The researchers envision a future where ships and submarines could carry aluminum pellets and a small amount of the gallium-indium alloy. By pumping in seawater and adding coffee, these vessels could generate hydrogen on demand to power their engines or fuel cells, eliminating the need for bulky hydrogen storage tanks.
While this technology holds great promise, several challenges must be addressed before it can be widely adopted. Notably, a detailed cost analysis comparing this method to other hydrogen production processes is essential to assess its economic viability.