Neuralink’s Blindsight device, designed to restore vision, has been granted “Breakthrough Device” designation by the US Food and Drug Administration (FDA). This classification doesn’t signify that the company has developed a cure for blindness but rather accelerates the development of this medical technology. Neuralink can now seek advice and assistance from FDA experts and benefit from expedited review processes.
Elon Musk has stated that this device could restore vision for individuals who have lost sight in both eyes, even if the optic nerve is damaged. Initially, the vision would resemble low-resolution graphics but could improve over time, potentially surpassing normal human vision.
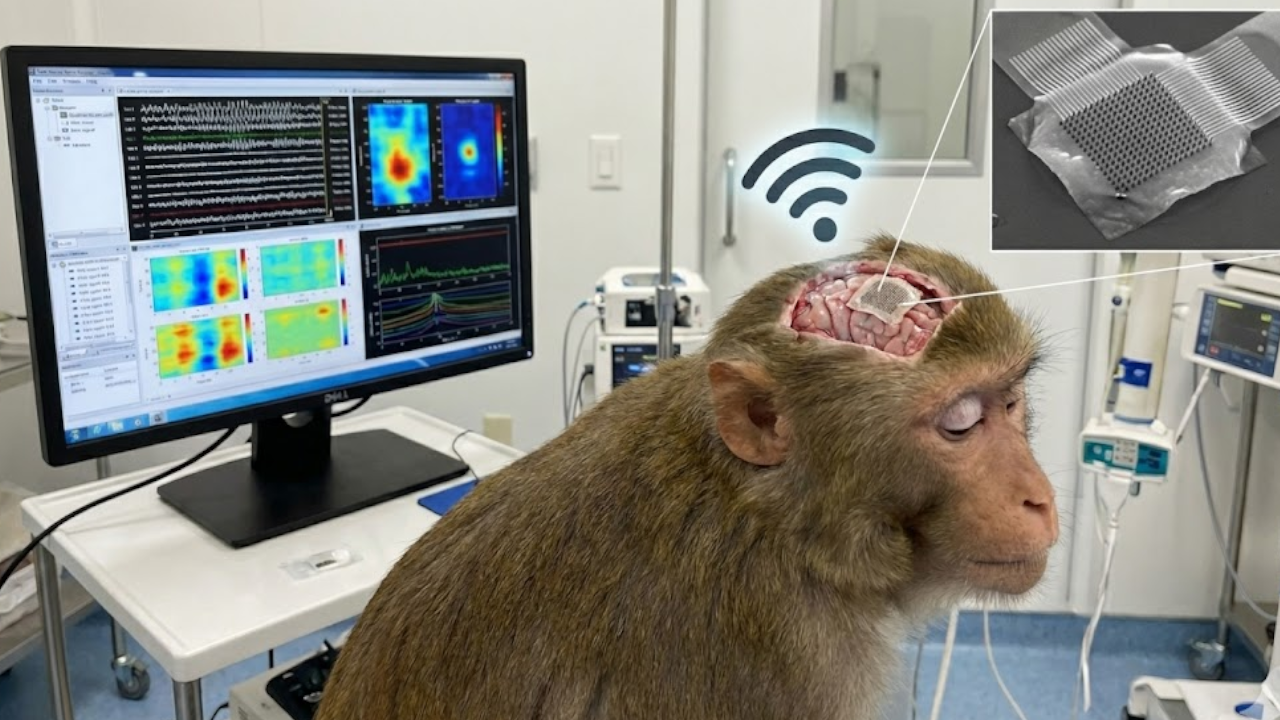
The device is a brain implant placed on the visual cortex, stimulating it with images from an external camera. This tricks the brain into perceiving these images as if they were coming from the optic nerve.
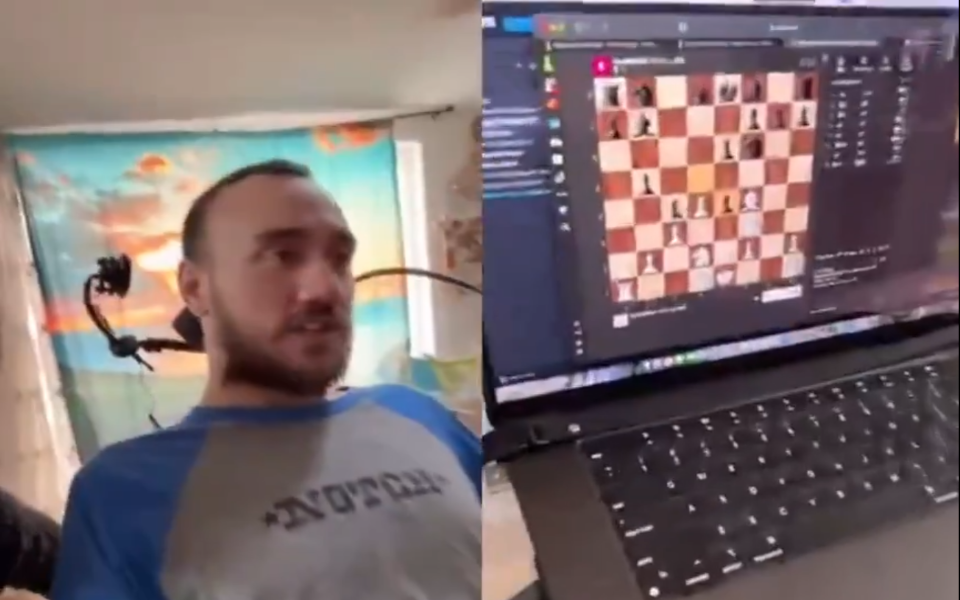
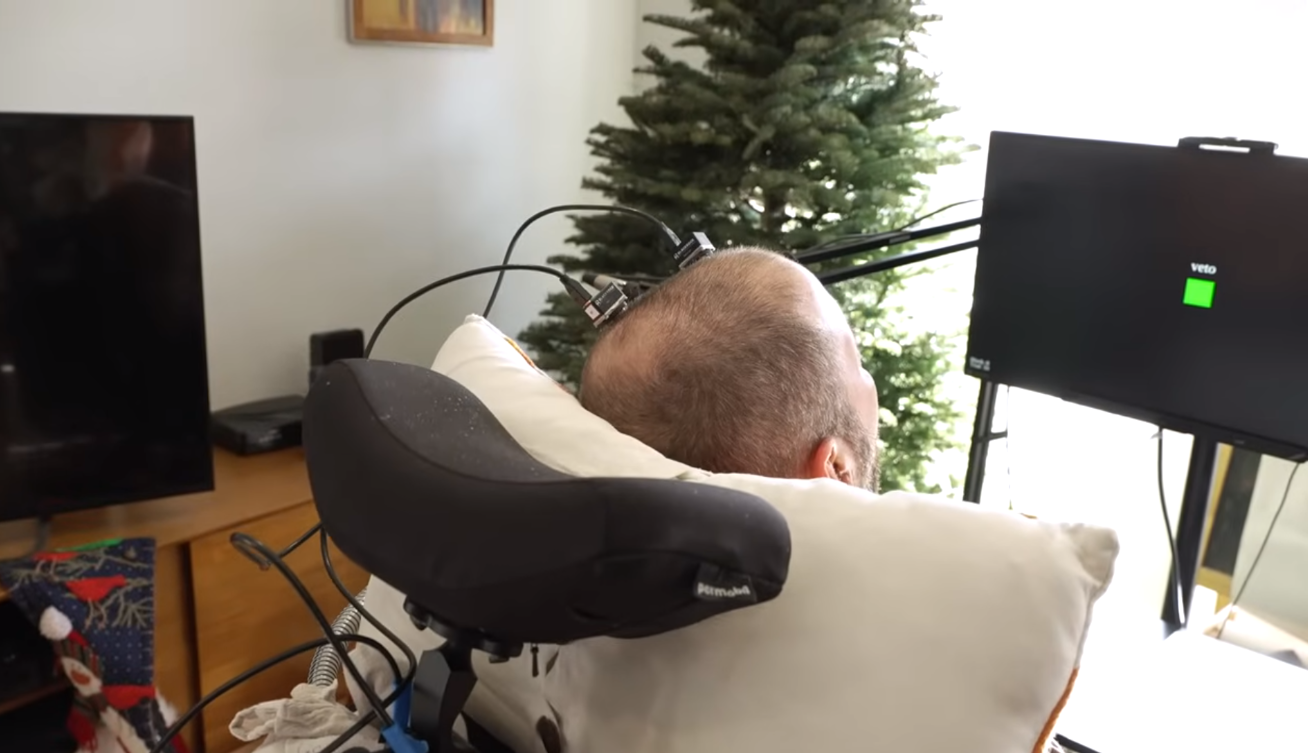
Neuralink previously achieved a significant milestone by implanting its device in two paralyzed patients who were able to play video games and use computers solely through thought, without vocal or physical input.
It is worth noting that Synchron, a competitor of Neuralink, recently succeeded in enabling a patient to control their smart home using Alexa through thought alone.